ASHP consensus report findings: Manufacturer premix systems rated highest1
In 2008, the Second Consensus Development Conference on the Safety of Intravenous Drug Delivery Systems was convened, organized by the American Society of Health-System Pharmacists. Its purpose was to reassess the safety of existing IV drug delivery systems since the implementation of new standards and guidelines developed after the first consensus conference in 1999. Changes that had occurred in the preceding decade—including the release of the United States Pharmacopeia (USP) chapter 797 guidelines for compounding sterile preparations. However, the safety of drug delivery and administration remained an area of concern, with “high-profile medication errors with IV medications occurring over the past few years….” Care was taken to assemble an independent expert panel that would provide “balanced, objective, and knowledgeable attention to the topic.” This panel produced the final consensus statement, based upon testimony of invited experts and subsequent discussions among all participants.
The goal of the conference was to develop a tool that could be used by practitioners to determine which drug delivery systems were the most appropriate for implementation at their facilities. The panel identified five IV drug delivery systems:
- manufacturer ready-to-use products
- products available from outsourcing vendors
- point-of-care activated products
- pharmacy-compounded products
- products that need manipulation at the point of care
Each was rated with a seven-point Likert scale on the basis of its score in each of six domains. A cumulative score was then calculated for each system and the drug delivery systems were ranked. The results are shown in the table below. (Roll over each domain to see the definition.)
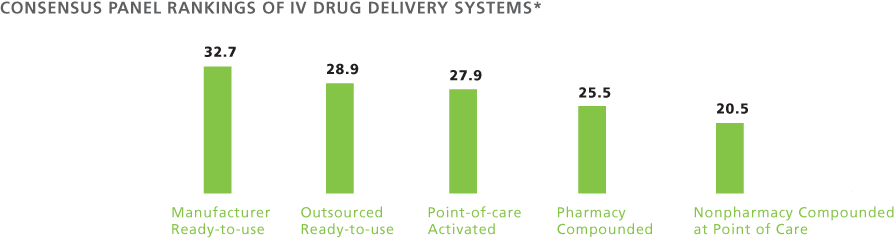
*Mean scores of rankings based on the Likert scale ranging from 1=very weak to 7=very strong
The panel also concluded that healthcare organizations continue to struggle with the integration of procuring and preparing IV medication products, the technology to assist with them, and personnel to provide the safest delivery and administration of IV medications to patients. The panel’s consensus was that every medication should be provided in the most ready-to-use form to the person responsible for administration.
1 Sanborn MD, Moody ML, Harder KA, et al. Second consensus development conference on the safety of intravenous drug delivery systems—2008. Am J Health-Syst Pharm. 2009;66:185-192.