Concentrate vials vs premix bags: 15 steps vs 7 steps
Premix bags are a simple and efficient way to help reduce the risk of medication errors.
Just compare the steps!
- The label is complete and free of errors.
- The correct IV solution, including size and strength, has been used.
- The correct drug additive, including strength and quantity, has been used.
- The admixture is clear and free from particulate matter.
- All drugs and solutions used are within their labeled expiration dates.
Personnel, Fifth Edition. Chicago, Ill.: Precept Press; 1995.
2 ZOLEDRONIC Acid Injection 4 mg Plastic Vial [package insert].
Schaumburg, Ill; Sagent Pharmaceuticals, Inc.; 2013.
TYPICAL ADMIX STEPS1,2
Steps for preparing an admixture:
- Wash your hands.
- Locate the ZOLEDRONIC Acid Injection vial AND the solution/bag you will use to dilute and administer zoledronic acid.
- Remove the plastic IV bag from its outer wrap (if necessary).
- Assemble the needle and syringe.
- Remove the protective cap from the zoledronic acid 4 mg per 5 mL vial and swab the top surface of the vial closure.
- Swab the medication port of the plastic bag with a disinfecting agent.
- Remove the protective cover from the syringe tip and insert the needle into the 4 mg per 5 mL vial.
- Withdraw 5 mL of the zoledronic acid solution from the vial and remove the needle from the vial.
- Insert the needle into the medication port and through the inner diaphragm of the IV bag.
- Inject the contents of the syringe into the IV bag and remove the needle from the bag.
- Properly dispose of the needle, syringe and vial in an appropriate SHARPS container and waste receptacle.
- Shake the bag and inspect the admixture to make sure it is clear and free of particulate matter.
- Label the 100 mL bag and document the time and date the admixture was prepared and the appropriate expiration time for the preparation.
- Locate an administration set.
- Insert the administration tubing spike into the 100 mL bag.
Schaumburg, Ill; Sagent Pharmaceuticals, Inc.; 2013.
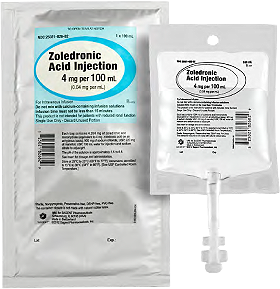
TYPICAL READY-TO-USE PREMIX BAG STEPS3
Steps for preparing a premix bag:
- Wash your hands.
- Locate the 4 mg per 100 mL ZOLEDRONIC Acid Injection ready-to-use premix bag.
- Remove the ready-to-use premix bag from the overwrap.
- Label the premix bag.
- Locate an administration set.
- Remove the protective cap from the medication port of the premix bag.
- Insert the administration tubing spike into the ready-to-use premix bag.
Everyday life’s essentials:
Progress marked by transitioning from long preparation to ready-to-use
![]()

See the Difference
Imagine if you had to use a hand churn every time you wanted butter
on your toast, pancakes or popcorn. Aren’t you glad butter comes in
a ready-to-use stick? By eliminating time-consuming process steps,
ready-to-use products can make life simpler and easier.
it all adds upSM

One of the ways in which SAGENT is striving to help caregivers reduce the risk of medication errors is by eliminating time-consuming and labor-intensive tasks involved with medication preparation. Ready-to-use premix bags do just that.
In a busy hospital pharmacy environment, the demands on the pharmacists’ time are immense. Between fielding STAT medication requests, calculating dosage information and balancing the ongoing needs and priorities required to manage the pharmacy, the last thing a pharmacist needs is a longer “to do” list. SAGENT’s premix bags — with easy-to-read labels, drug names and dose information — can help lighten the load because no admixing steps are necessary. Less (time spent on med prep) really IS more.
That’s PreventIV Measures Packaging and Labeling at work for you.